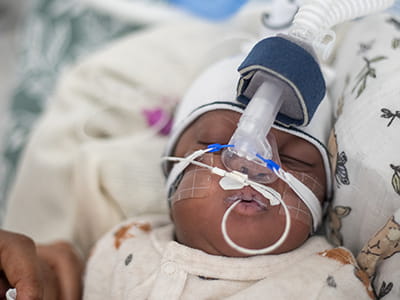
NICU point-of-care testing solution
Enhancing your ability to monitor the oxygenation-ventilation balance, with minimal blood draws.
-
 Minimise invasive procedures and blood draws
Minimise invasive procedures and blood draws
-
 Continuous, real-time monitoring of CO2 and O2
Continuous, real-time monitoring of CO2 and O2
-
 Combine blood gas analysis with transcutaneous monitoring
Combine blood gas analysis with transcutaneous monitoring
Monitoring a neonate´s oxygenation-ventilation balance
To much or too little CO2 and O2 can lead to severe challenges for the baby.
Blood gas analysis provides the most precise measures of CO2 and O2, but blood sampling is invasive and can contribute to the risk of neonatal anemia.
Transcutaneous monitoring provides a continuous trend of CO2 and O2, and exhibits strong correlation well with arterial values.Patient Blood Management
 A preterm baby born weighing just 500 grams has an estimated total blood volume of less than 50 ml.
A preterm baby born weighing just 500 grams has an estimated total blood volume of less than 50 ml.Blood sampling can deplete 30%-60% of the total neonatal blood volume in the first weeks of life.
Preterm babies endure an average of 50 painful procedures during their first four weeks of life.
Click here to renew consent
Blood gases are critical to titrating therapies and ventilation. If we don't do blood gas analyses, we provide 'blind'care, 'blind' therapies, that could negatively influence babies' brain development. We need to find a balance, which is why a wise doctor could use both non-invasive transcutaneous monitoring, alongside minimally invasive therapies such as blood gas analyses, using capillary samplers.
- Daniele De Luca, Full Professor of Neonatology and Division Chief, Antoine Béclère Hospital, Paris Saclay University, Paris, France
Blood gases are critical to titrating therapies and ventilation. If we don't do blood gas analyses, we provide 'blind'care, 'blind' therapies, that could negatively influence babies' brain development. We need to find a balance, which is why a wise doctor could use both non-invasive transcutaneous monitoring, alongside minimally invasive therapies such as blood gas analyses, using capillary samplers.
- Daniele De Luca, Full Professor of Neonatology and Division Chief, Antoine Béclère Hospital, Paris Saclay University, Paris, France
Click here to renew consent
Every drop of blood counts - complement blood gas testing with transcutaneous monitoring
Blood gas analysis reflects a patient's ventilation (CO2) and oxygenation (O2) status at a single point in time.
Supplementing blood gas analysis with continuous monitoring of transcutaneous oxygen and carbon dioxide can conserve babies' blood and minimise invasive blood draws as well as facilitate proactive patient care - by providing a constant trend for both blood gases.
Transcutaneous monitoring
- Exhibits strong correlation with arterial values
- Can be used on babies with a very low birth weight (<1000g)
- Can be used on babies that are extremely premature (<28 weeks)
- Continuously trends CO2 and O2
We use transcutaneous monitoring (TCM) almost every day to tailor ventilation, check how a baby is generally and to try and conserve as much blood as is possible. Of course, we also conduct blood gas analyses, but TCM provides an opportunity to reduce blood gas analyses overall.
- Daniele De Luca, Full Professor of Neonatology and Division Chief, Antoine Béclère Hospital, Paris Saclay University, Paris, France
Explore the Radiometer NICU solution

Sample Size and Time to Result
17 parameters:
45ul blood / 60 secs.
or
19 parameters (incl. creatinine/urea):
65ul blood / 35 secs.
Bilirubin measure
Measure bilirubin to help identify and monitor the development of hyperbilirubinemia.
Automatic Quality Management (AQM)
Automatic Quality Management system facilitates reliable results.
Clot detection
Advanced clot detection system so micro and macro clots are automatically detected and removed.
70ul capillary tube made of plastic
70ul of blood / 35 secs:
19 parameters:
Blood gases: pH, pCO2, pO2
Metabolites: cGlu, cLac, cCrea, cUrea
Electrolytes: cCa2+, cCl-, cK+, cNa+
Oximetry: FCOHb, ctBil, ctHb, FHbF, FHHb, FMetHb, sO2, FO2Hb
45ul capillary tube made of plastic
45ul of blood / 60 secs.
17 parameters:
Blood gases: pH, pCO2, pO2
Metabolites: cGlu, cLac
Electrolytes: cCa2+, cCl-, cK+, cNa+
Oximetry: FCOHb, ctBil, ctHb, FHbF, FHHb, FMetHb, sO2, FO2Hb
ABL90 FLEX PLUS blood gas analyser
Achieve comprehensive insights from less than two drops of blood.
- 45ul MicroMode designed for the realities of neonatal patients' status
- safeCLINITUBES help to minimise the risk of preanalytical errors, such as clot formation.
ABL90 FLEX PLUS blood gas analyser

Seamless connectivity
Seamless connectivity into a wide range of monitoring and data management systems and ventilators.
Automatic calibration
Calibrations automatically start when placing the sensor in the calibration chamber.
Real-time data
Real-time data provides full transparency on babies' status between blood draws.
Dedicated NICU mode
A dedicated NICU mode which locks the monitoring time to the sensor temperature, protecting the babies' skin integrity.
Large color touchscreen
A large color touchscreen for clear data display and built-in tutorials supporting a system that is easy to use.
Designed for sensitive skin
Continuously measure tcpCO2 and tcpO2 utilizing a variety of patient fixation options designed for sensitive skin.
TCM5 FLEX transcutaneous monitor
Supplementing blood gas analysis, aiming to reduce sampling and risk of anaemia - and be proactive with patient care.
- Established transcutaneous technology to measure both CO2 and O2
- Gentle sensor with a variety of fixation options designed for sensitive skin.
Parameters measured with the Radiometer NICU solution
ABL90 FLEX PLUS: pH | pCO2 | pO2 | cGlu | cLac | cCrea | cUrea | cCa2+ | cCl- | cK+ | cNa+ | FCOHb | ctBil | ctHb | FHbF | FHHb | FMetHb | sO2 | FO2Hb
TCM5 FLEX: tcpCO2 | tcpO2 | Pulse rate
Parameters measured with the Radiometer NICU solution
ABL90 FLEX PLUS: pH | pCO2 | pO2 | cGlu | cLac | cCrea | cUrea | cCa2+ | cCl- | cK+ | cNa+ | FCOHb | ctBil | ctHb | FHbF | FHHb | FMetHb | sO2 | FO2Hb
TCM5 FLEX: tcpCO2 | tcpO2 | Pulse rate
Learn more about the role tcpCO2 and tcpO2 monitoring can play in the NICU

Importance of invasive & non-invasive CO2 monitoring in the NICU
Oxygen monitoring in the NICU
Blood gas monitoring in the preterm neonate
Integrated POC IT and Services - Connect & Care
Connect your people, medical devices and data.
Connect & Care offers you sustainable support from certified specialists, as well as a range of services for the lifetime
of your solution.
We go beyond practical support, offering you peace of mind, and positive experiences for patients and their loved ones.
Cookies are used on this website
Use of cookiesPlease enter a valid email
We will be sending an e-mail invitation to you shortly to sign in using Microsoft Azure AD.
It seems that your e-mail is not registered with us
Please click "Get started" in the e-mail to complete the registration process
Radiometer is using Microsoft AZURE Active Directory to authenticate users
Radiometer uses Azure AD to provide our customers and partners secure access to documents, resources, and other services on our customer portal.
If your organization is already using Azure AD you can use the same credentials to access Radiometer's customer portal.
Key benefits
- Allow the use of existing Active Directory credentials
- Single-sign on experience
- Use same credentials to access future services
Request access
You will receive an invitation to access our services via e-mail when your request has been approved.
When you accept the invitation, and your organization is already using AZURE AD, you can use the same credentials to access Radiometer's customer portal. Otherwise, a one-time password will be sent via e-mail to sign in.
